DeltaStudy successfully completed
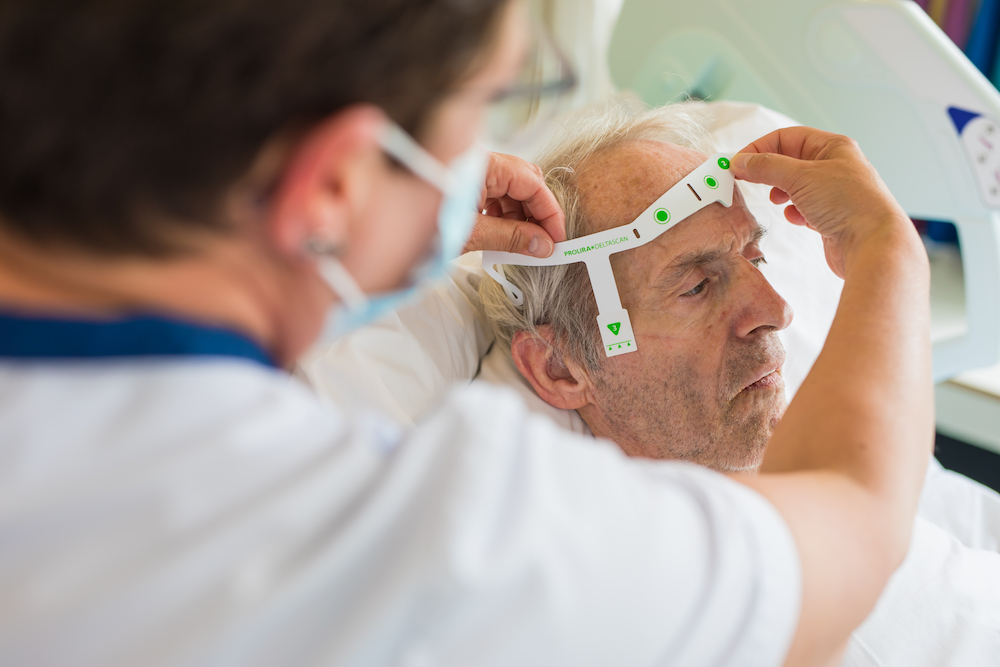
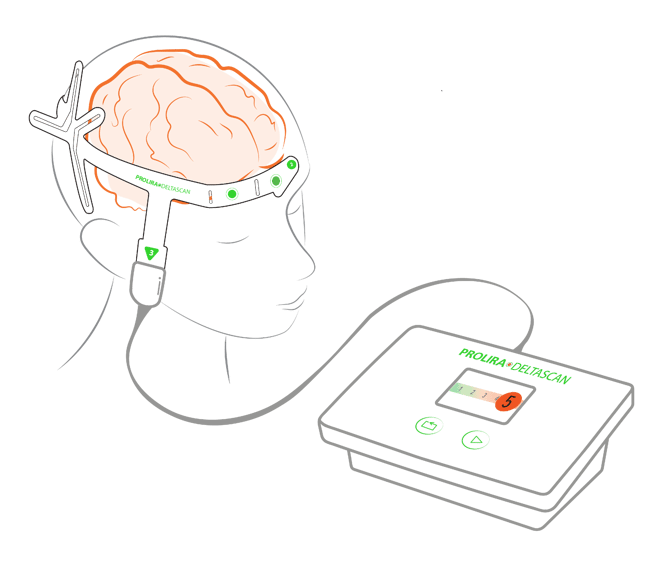
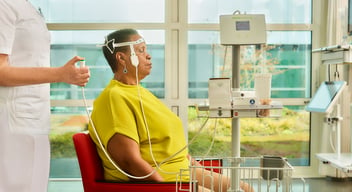
In this multicenter study, 434 patients measured with DeltaScan. A large scale validation study for DeltaScan device performance was executed. Patient data was collected from a wide study population of 434 patients. The aim of the study was to investigate the diagnostic performance of the DeltaScan, a CE-certified device to detect acute encephalopathy and delirium using a brief electroencephalography (EEG) recording, separately in patients admitted to an Intensive Care Unit (ICU), and elderly patient admitted to the ward (non-ICU department).
Cross-sectional, multicentre study in 9 hospitals
The design of the study was a Cross-sectional, multicentre study in 9 hospitals with 31 different departments; nearly 50% of the patients were in the ICU, the better half of the patients were in a variety of wards (e.g. cardiothoracic surgery, geriatric traumatology, geriatrics, internal medicine, etc). The study results show strong performance (>85% overall diagnostic accuracy) of DeltaScan for acute encephalopathy (a.k.a. delirium) in the variety of hospital departments.