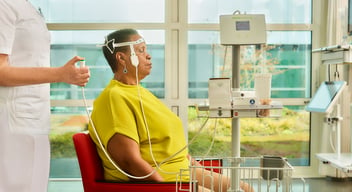
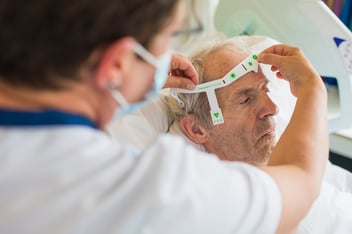
EU MDR certification for Prolira DeltaScan!

Regulatory Milestone: Prolira has EU MDR certification! We are proud to announce that Prolira is one of the first medical device companies in the world, to be certified according to the new EU MDR.
Prolira’s EU MDR certifiction is the result of a big team effort headed by Uschi and Rutger. We also wish to thank QAid for its full support and DARE!! Medical Certifications for the effective and constructive auditing and certification process.
The European Medical Device Regulation (EU MDR) guarantees safety and quality requirements for medical devices being manufactured or imported into Europe. It underwent fundamental revisions in 2017 to improve transparency through standard data, technological advances and the creation of an EU (EUDAMED)database. While the MDD was simply a set of guidelines, MDR is legally enforceable by EU member states.